Click on the title for a link to the paper
Selected Publications
Intrinsic Muscle Stem Cell Dysfunction Contributes to Impaired Regeneration in the mdx Mouse
Journal of Cachexia, Sarcopenia and muscle 2025
Esper et al., provide evidence that dystrophin deficiency in MuSCs and myofibres together contributes to progression of DMD. Ongoing muscle damage stimulates MuSC activation; however, aberrant intrinsic MuSC polarity and stem cell commitment deficits due to the loss of dystrophin impair muscle regeneration. The study provides in vivo validation that dystrophin-deficient MuSCs undergo fewer asymmetric cell divisions, instead favouring symmetric expansion.
Identification of the Wnt signal peptide that directs secretion on extracellular vesicles
Science Advances 2024
In this work by Gurriaran-Rodriguez et al., we discovered the exosome-targeting postal code or zip code within a protein called Wnt7a, which plays a critical role in development, growth, regeneration and cancer. First, we showed that Wnt7a can attach itself to exosomes. Then, we deleted various parts of the Wnt7a protein until we found the smallest part that was responsible for exosome-targeting. We called this part, which consists of 18 amino acids, the Exosome Binding Peptide (EBP). We then discovered that the EBP binds to proteins called Coatomers on exosomes, and that EBP could be used to target any protein to exosomes.
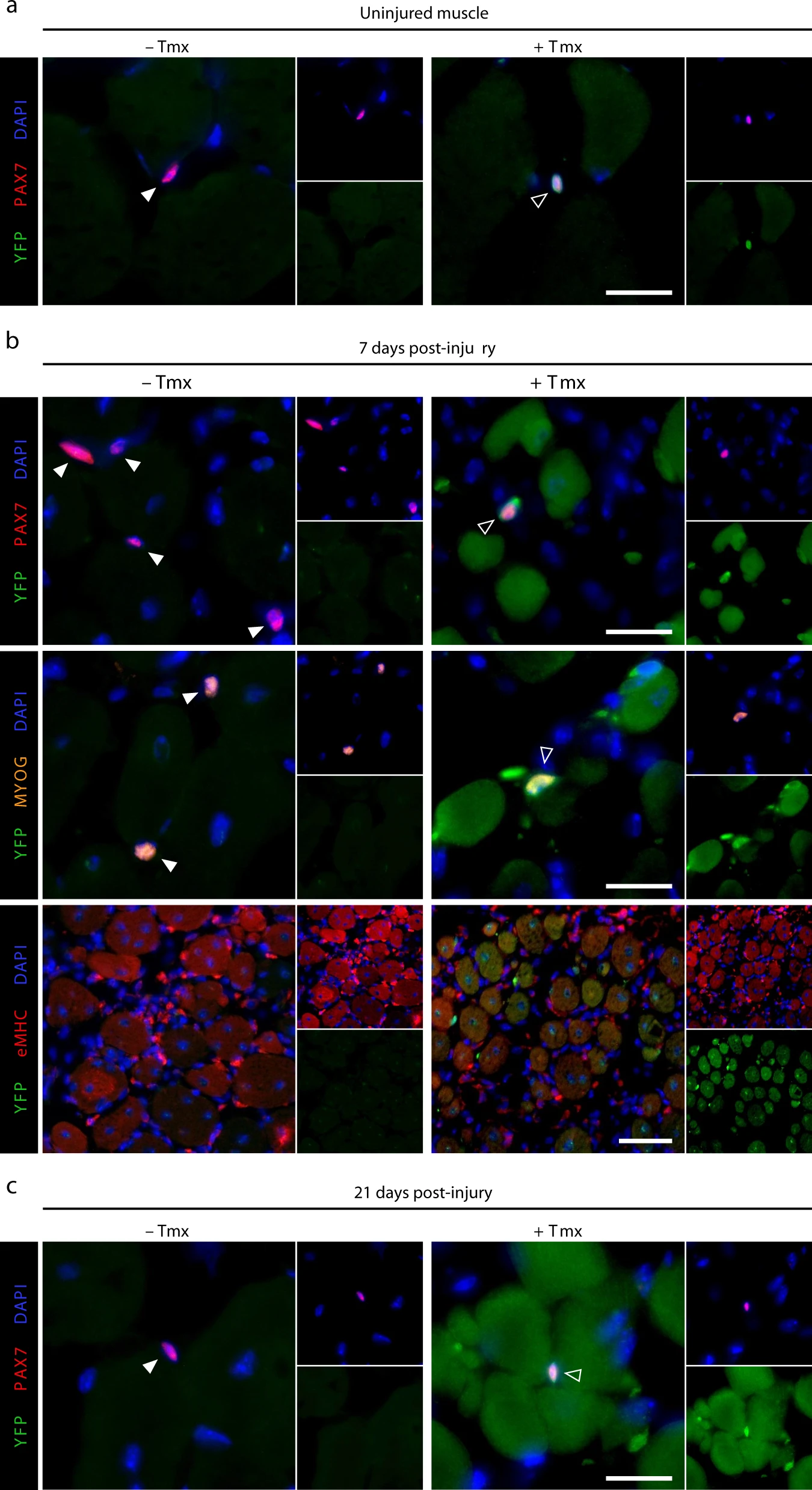
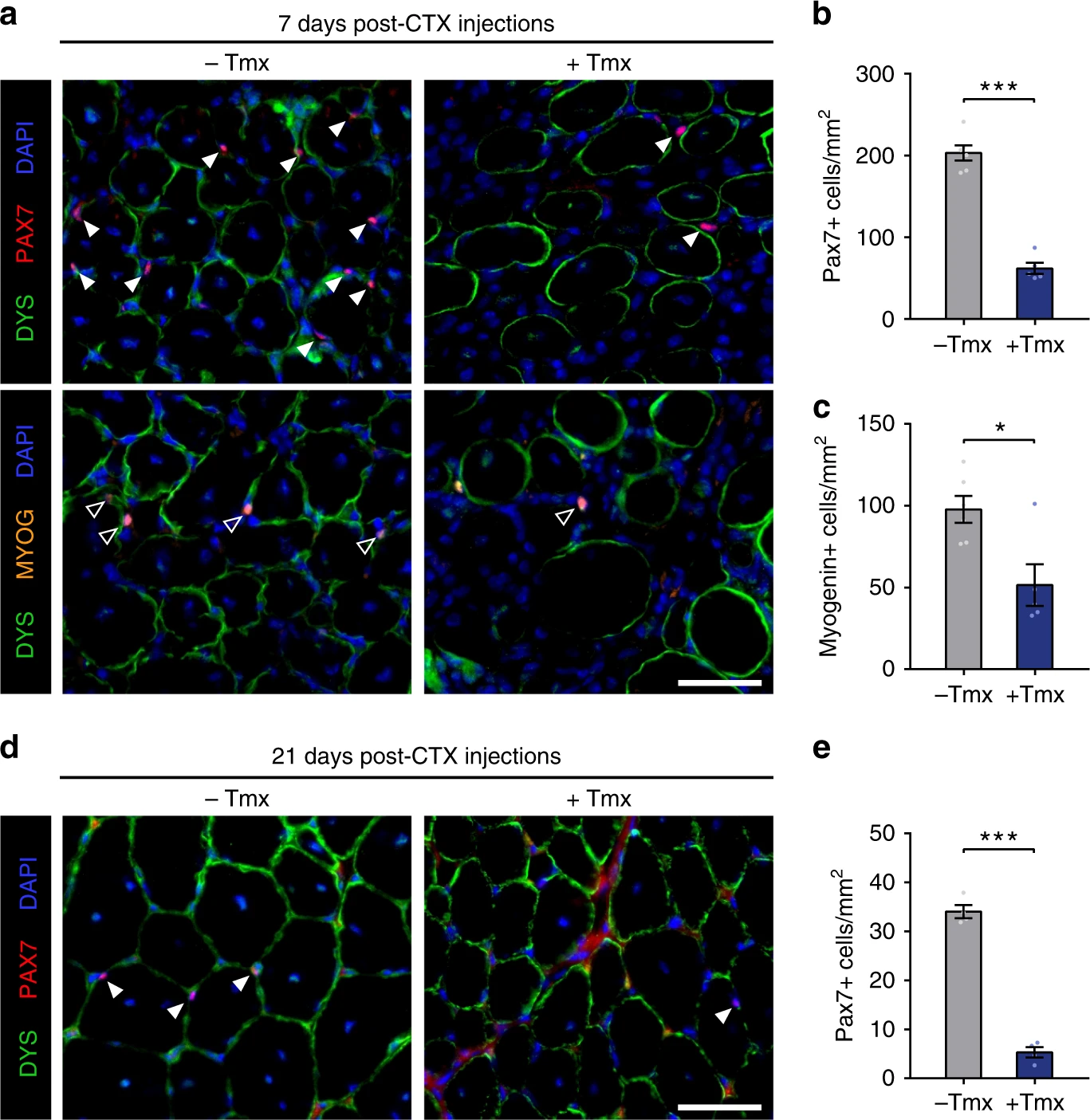
Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division
Nature Medicine 2015
Dumont et al. demonstrate that dystrophin is expressed in activated satellite cells, in which it regulates polarity establishment by interacting with Mark2. Dystrophin-deficient satellite cells show impaired polarity establishment, loss of apicobasal asymmetric division and a higher proportion of abnormal division, leading to reduced generation of myogenic progenitors and impaired muscle regeneration.
MLL1 is required for PAX7 expression and satellite cell self-renewal in mice
Nature Communications 2019
In this study, Addicks et al. uncover a specific role of MLL1 in regulating PAX7 expression, elucidating their involvement in Myf5 transcriptional control. Using conditional alleles of Mll1 and Mll2, we find that MLL1 directly regulates Pax7. In the absence of Mll1, myoblasts display reduced H3K4me3 enrichment at both Pax7 and Myf5 loci associated with the loss of Pax7 and Myf5 expression. As a consequence, Mll1-deficient myoblasts fail to proliferate but retain their potential to differentiate. Re-establishing PAX7 expression in committed Mll1 cKO myoblasts is sufficient to restore H3K4me3 enrichment at the Myf5 promoter and rescue Myf5 levels, indicating that PAX7, but not MLL1, is required for Myf5 expression in committed myoblasts.
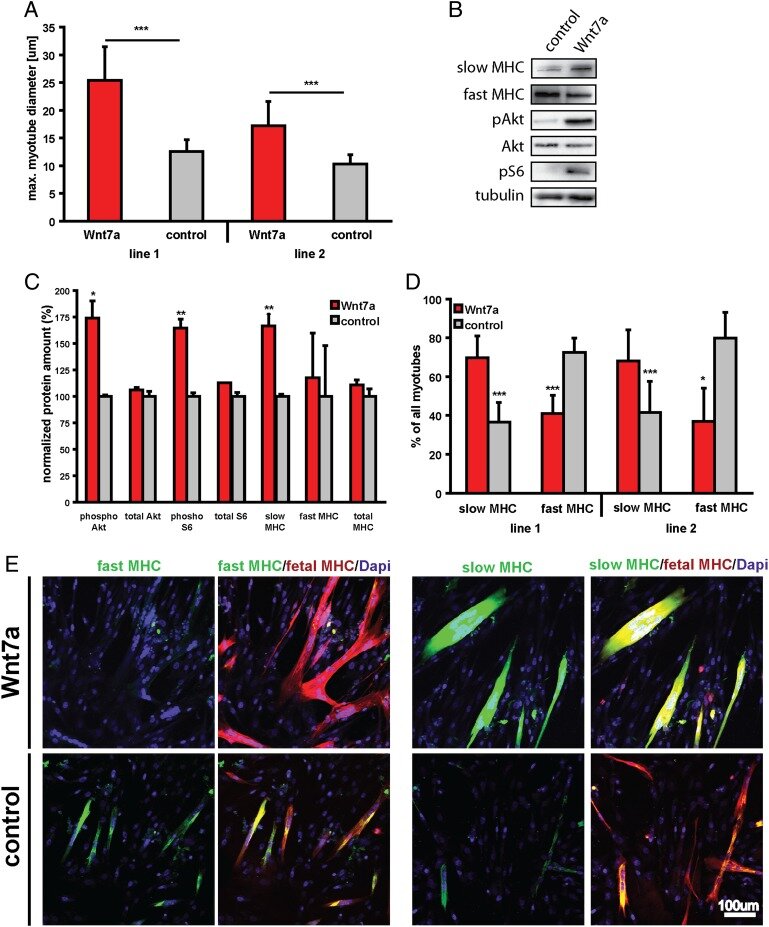
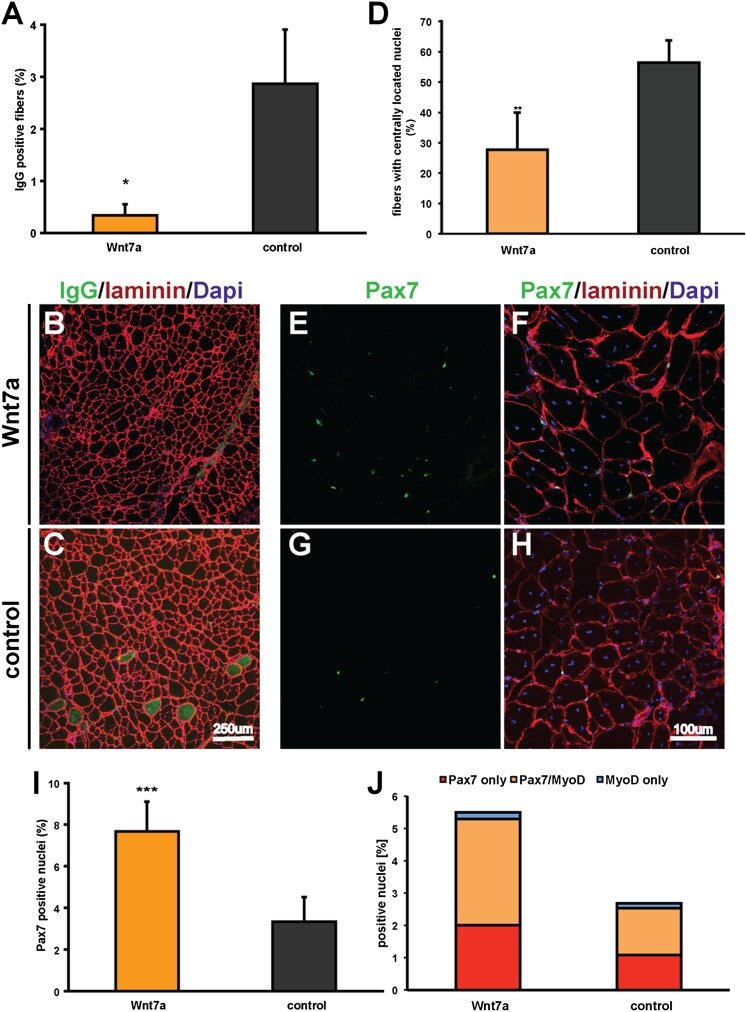
Wnt7a treatment ameliorates muscular dystrophy
PNAS 2012
Von Maltzahn et al. have established that Wnt7a/Fzd7 signaling acts at two levels to regulate muscle homeostasis. Wnt7a activity couples muscle growth with stem cell expansion, leading to productive hypertrophy. The ability of Wnt7a to drive these two different pathways in skeletal muscle suggests that Wnt7a may be used as a therapeutic approach to stimulate regeneration of dystrophic muscles in DMD.
EGFR-Aurka Signaling Rescues Polarity and Regeneration Defects in Dystrophin-Deficient Muscle Stem Cells by Increasing Asymmetric Divisions
Cell Stem Cell 2019
Here we report the identification of epidermal growth factor receptor (EGFR) and Aurora kinase A (Aurka) pathways as determinants of asymmetric satellite stem cell divisions through an in-niche muscle stem cell screen. EGF stimulation activates EGFR localized at the basal surface of muscle stem cells and recruits the mitotic spindle assembly protein Aurka to induce apicobasal asymmetric divisions. Knockdown of Aurka abolishes EGF-induced asymmetric divisions. Importantly, the EGFR polarity pathway acts independently of dystrophin and can rescue the deficit in asymmetric division in dystrophin-deficient satellite cells.
The Dystrophin Glycoprotein Complex Regulates the Epigenetic Activation of Muscle Stem Cell Commitment
Cell Stem Cell 2018
In this paper, Chang et al. identify a role for the mitogen-activated protein kinase (MAPK) p38γ/MAPK12 as a critical regulator of satellite stem cell fate through phosphorylation of Carm1. They have defined an epigenetic regulatory pathway that mediates commitment during asymmetric division acting downstream of the DGC and polarity establishment. They also suggest that these signaling pathways represent an additional deficit owing to the lack of dystrophin expression in satellite cells, thus impairing the function of satellite cells in DMD.
Pax7 is required for the specification of myogenic satellite cells
Cell 2000
Seale et al. identified several myoblast-specific genes potentially involved in satellite cell function. Pax7 was selected for further analysis based on the established role of the closely related Pax3 protein in regulating the developmental program of embryonic myoblasts. In this article, they demonstrate a unique requirement for Pax7 in the specification of myogenic satellite cells.
For all publications, click here